Copyright © 2026. All rights reserved.
Website design and development by Mole Digital
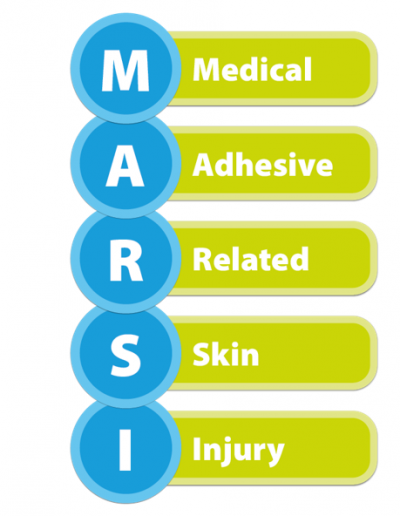
What is MARSI?
Medical Adhesive-Related Skin Injury (MARSI) occurs when medical adhesives remove superficial layers of skin, resulting in variable levels of skin damage, such as skin-stripping, tension blisters, skin tears, contact dermatitis1.
MARSI can occur to any group of patients in any setting and is often under-recognised as a skin issue – possibly because it is so common.



 Success!
Success!